博文
意外的化学反应可能提供新的工业应用
 精选
精选
||
意外的化学反应可能提供新的工业应用
诸平
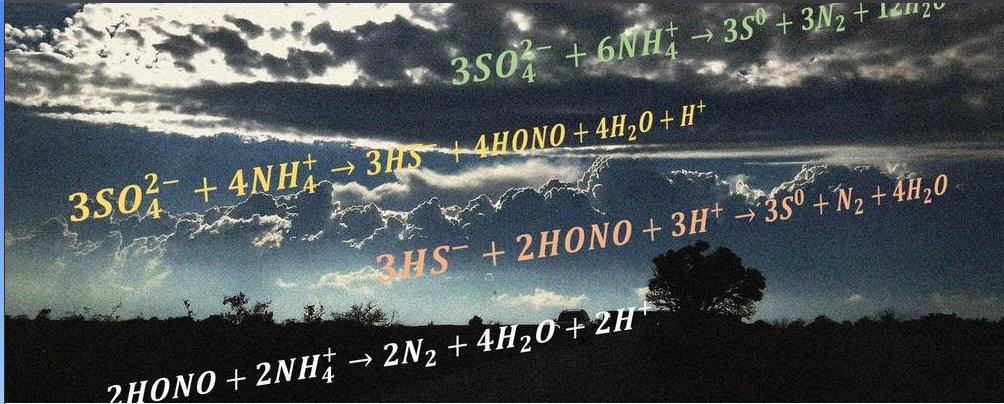
据瑞典歌德堡大学(University of Gothenburg)2021年11月9日提供的消息,《科学》(Science)杂志的一项新研究提出了新发现的涉及水溶性粒子表面的化学反应。由哥德堡大学的一个团队发起的这项研究可能在未来对污水处理有用(Unexpected discovery may offer new industrial applications)。
气体和气溶胶(aerosol)颗粒对大气中的化学物质起着重要作用。它们的相互作用影响着云和气候。气溶胶的典型例子是烟、雾和空气污染。气溶胶粒子尤其重要,即使它们不可见,因为它们的表面在大气中,不同的是只由气体组成。一项新的研究发现,当这些表面开始吸水和溶解时,新的和以前未发现的化学物质就会发生。
新化学的惊人发现(Surprising discovery of new chemistry)
2021年11月5日在《科学》(Science)杂志上发表了一篇新文章——Xiangrui Kong, Dimitri Castarède, Erik S. Thomson, Anthony Boucly, Luca Artiglia, Markus Ammann, Ivan Gladich, Jan B. C. Pettersson. A surface-promoted redox reaction occurs spontaneously on solvating inorganic aerosol surfaces, Science, 5 Nov 2021, Vol 374, Issue 6568, pp. 747-752. DOI: 10.1126/science.abc5311,在此文中,研究人员描述了当硫酸铵的表面溶解时,它会促进硫酸盐还原铵的氧化。这一发现令人惊讶,因为这种反应通常需要额外的能量来克服势垒,因此不会自发发生。
哥德堡大学(University of Gothenburg)研究者、该研究的主要作者孔祥瑞(Xiangrui Kong音译)说:“看到此反应发生,我们很惊讶,但我们逐渐了解到,水溶性盐表面使反应成为可能。这让我们重新思考表面的催化作用,在合适的条件下,一些反应实际上是可以被促进的。”
工业和环境应用的可能效益(Possible benefits for cost-effective industrial and environmental applications)
这项研究对我们理解大气硫循环和大气中许多化学反应中涉及的其他重要化合物的发生有影响。例如,所观察到的硫酸盐还原氨氧化反应也可用于污水处理和其他工业应用。目前需要生物催化剂或其他昂贵的方法来激活这种反应。研究人员表示,这种新的机制表明,基于这种新知识,可能会开发出具有成本效益的应用。
该研究的作者之一、哥德堡大学高级讲师埃里克·汤姆森(Erik S. Thomson)说:“当我们开始意识到我们的结果时,我们花了很长时间来理解我们观察到的东西和为什么,但结果令人兴奋,并激励我们深入挖掘表面相变如何改变化学环境。”
上述介绍,仅供参考。欲了解更多信息,敬请注意浏览原文或者相关报道。
Spontaneous chemistry on aerosol surface
Interfacial redox chemistry plays an important role in the formation of gas molecules and aerosol particles. However, the characterization of such heterogeneous processes is challenging, and they are often omitted in chemical kinetics models. Using ambient-pressure x-ray photoelectron spectroscopy combined with molecular dynamics simulations, Kong et al. discovered spontaneous redox chemistry promoted by the surface at the first stages of the solvation process on a typical inorganic aerosol surface of ammonium sulfate (see the Perspective by Ruiz-Lopez). Several unexpected species have been identified as being possible products of a sulfate-reducing ammonium oxidation reaction, and this may help to resolve some of the enduring conundrums of atmospheric chemistry. The present results could also be useful for the development of wastewater treatments and other industrial technologies. —YS
A surface-promoted sulfate-reducing ammonium oxidation reaction was discovered to spontaneously take place on common inorganic aerosol surfaces undergoing solvation. Several key intermediate species—including elemental sulfur (S0), bisulfide (HS−), nitrous acid (HONO), and aqueous ammonia [NH3(aq)]—were identified as reaction components associated with the solvation process. Depth profiles of relative species abundance showed the surface propensity of key species. The species assignments and depth profile features were supported by classical and first-principles molecular dynamics calculations, and a detailed mechanism was proposed to describe the processes that led to unexpected products during salt solvation. This discovery revealed chemistry that is distinctly linked to a solvating surface and has great potential to illuminate current puzzles within heterogeneous chemistry.
https://blog.sciencenet.cn/blog-212210-1311716.html
上一篇:光伏太阳能加热系统使用95%的可用能源来加热水
下一篇:使用新的量子计算架构来创造时间晶体