博文
Triphenylamine-AIE Materials for Cancer Theranostics
|
Triphenylamine-AIEgens Photoactive Materials for Cancer Theranostics
Junjie Wang【王俊杰】c#, Yan Wanga,b#, Zhengdong Lie#, Changqiang Xiea,b, Mussmmir Khanf, Xingzhou Peng【彭星舟】a,b*, Fabiao Yu【于法标】c,d*
a State Key Laboratory of Digital Medical Engineering, School of Biomedical Engineering, Hainan University, Haikou 570228, China
b Key Laboratory of Biomedical Engineering of Hainan Province, One Health Institute, Hainan University, Haikou 570228, China
c Key Laboratory of Hainan Trauma and Disaster Rescue, The First Affiliated Hospital of Hainan Medical University, Hainan Medical University, Haikou 571199, China
d Engineering Research Center for Hainan Bio-Smart Materials and Bio-Medical Devices, Key Laboratory of Emergency and Trauma, Ministry of Education, Key Laboratory of Hainan Functional Materials and Molecular Imaging, College of Emergency and Trauma, Hainan Medical University, Haikou 571199, China
e Precision medical center, Guangyuan City Centre hospital, Guangyuan 628099, China
f School of Natural Sciences (SNS), National University of Science and Technology (NUST), H-12, Islamabad 44000, Pakistan
https://doi.org/10.1016/j.cclet.2023.108934
Graphical abstractThe photoactive agents based on triphenylamine-based aggregation-induced emission luminogens (TPA-AIEgens) for cancer theranostics were summarized, including the design principle, targeting strategies, and the latest progress in biological imaging and cancer treatment.

Triphenylamine (TPA)-based aggregation-induced emission luminogens (TPA-AIEgens), a type of photoactive material utilizing the typical TPA moiety, has recently attracted increasing attention for the diagnostics and treatment of tumors due to their remarkable chemo-physical performance in optoelectronic research. TPA-AIEgens are distinguished from other photoactive agents by their strong fluorescence, good sensitivity, high signal-to-noise ratio, resistance to photobleaching, and lack of high concentration or aggregation-caused fluoresce quenching effects. In this review, we summarize the current advancements and the biomedical progress of TPA-AIEgens in tumor theranostics. First, the design principles of TPA-AIEgens photoactive agents as well as the advanced targeting strategies for nuclei, cell membranes, cell organelle and tumors were introduced, respectively. Next, the applications of TPA-AIEgens in tumor diagnosis and therapeutic techniques were reviewed. Last, the challenges and prospects of TPA-AIEgens for cancer therapy were performed. The given landscape of the TPA-AIEgens hereby is meaningful for the further design and utilization of the novel photoactive material, which could be beneficial for the development of clinic applications.
Malignant tumors are the primary cause of human mortality, which represent a severe threat to human health and survival [1]. Nowadays, surgery, radiation, and chemotherapy are the three primary traditional treatments. Unfortunately, these traditional treatments suffer defects, such as significant systemic side effects and high recurrence rates [2,3]. Recently, phototheranostics have made significant contributions to cancer treatment due to their light-controllability, non-invasiveness, specific target, low toxicity, and good selectivity [4-6]. Normally, photodynamic therapy (PDT) and photothermal therapy (PTT) are two types of phototherapies that exogenous photosensitizing agents are involved to selectively kill tumors or inhibit tumor growth with light radiation [7,8]. In PDT, photosensitizers (PSs) are typically used to generate cytotoxic reactive oxygen species (ROS), such as singlet oxygen (1O2), superoxide (O2–-) and hydroxyl radical (–OH), which is able to eliminate tumor cells [5,9]. Photothermal agents can enhance the heating process of cells and tissues in the local area by absorbing laser energy and converting it into heat [10-12], which is similar to laser therapy [13]. The main discrepancy is that laser therapy uses endogenous chromophores with non-selectivity towards malignant cells, while PTT with exogenous agents can specifically target cancer cells. With the aid of photoactive agents, both the PDT and PTT can produce vigorous interaction with biological substances, leading to the immunogenic cell death (ICD), vascular injury, and immunological response [14-17].
Recently, the photoactive agents based on AIE luminogens (AIEgens) developed by Tang Benzhong's research group have made breakthroughs in phototherapy research. An interesting phenomenon termed aggregation-induced emission (AIE) occurs when a series of non-emissive molecules in the dispersed state are induced to show strong emission upon aggregate formation or in solid state [18]. Due to the existence of the strong electron-vibration coupling in the dispersed state, AIE molecules demonstrate the intrinsic non-radiative transitions and fluorescence quench. In the aggregated state, the molecular environment's restriction of intramolecular movements (RIM) might impair the electron-vibrational coupling of molecular system, resulting in depressed non-radiative transitions and enhanced fluorescence [19,20]. In general, the clinical diagnostic agents (e.g. ICG, porphyrin) suffer from certain problems such as aggregation-induced fluorescence quenching (ACQ) and photobleaching [21,22]; while AIEgens offers the following benefits compared to the traditional fluorescent dyes: 1) high-intensity fluorescence; 2) efficient light conversion; 3) strong stability; 4)luminescence regulation by flexible chemical modification; 5) high resolution in biological imaging [23,24].
The fundamental AIE motifs, such as tetraphenylene (TPE), triphenylamine (TPA), 2,3,4,5-tetraphenylsiloles (TPS), phenylvinyl anthrance, and phenyl substituted pyrrole, are integral to the creation of AIE materials [25]. In contrast to typical TPE-AIEgens, TPA-AIEgens is more flexible to be designed for in vivo applications such as high water-solubility and strong NIR II absorption. The chemical structure of TPA is composed of three benzene rings and a central nitrogen atom. Due to its unique helical structure and three phenyl rotors, TPA not only plays a role as the strong electron donor but also acts as a molecular rotor, making it easy to construct a variety of TPA-AIEgens. As an electron donor, TPA is combined with an electron acceptor to obtain a molecule with donor-acceptor (D-A) structure resulting in an adjusted intramolecular push-pull electron interaction, which benefits the red-shift of fluorescence and/or enhances intersystem crossing (ISC) process to generate more ROS [25,26]. When acting as a molecular rotor, the highly distorted conformation of TPA increases the molecular distance, reducing the intermolecular π-π packing and retaining the intramolecular rotation, which is beneficial to promote fluorescence and/or heat generation [27,28]. Therefore, TPA is a crucial functional segment in the construction of TAP-AIEgens.
Up to now, TPA-AIEgens have been developed as novel molecular materials with photosensitive and photothermal capabilities, demonstrating the huge potential in tumor theranostics [29]. Thus, the design principles, targeting strategies, and the latest progress in biological imaging and treatment based on TPA-AIEgens photoactive agents are summarized in this minireview (Scheme 1). First, the principles of design TPA-AIEgens with near-infrared absorption and good biocompatibility were summarized. Next, TPA-AIEgens targeting strategies, including targeting to nuclei, cell membranes, cell organelles and tumors, were presented. Then it introduced the application of TPA-AIEgens in biological imaging and phototherapy for the most recent years. Finally, the photoactive agents based on TPA-AIEgens for cancer treatment were prospected. We hope this review can provide valuable information for design and application of TPA-AIEgens to accelerate their clinical translation.

Scheme. 1. TPA-AIEgens for cancer theranostic, including targeting strategies, biological imaging and cancer treatment.
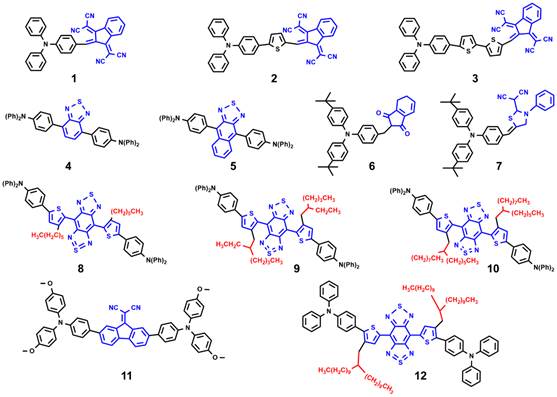
Fig. 1. Typical TPA-AIEgens used for cancer diagnosis and treatment. Compound 1-3 with extended π system to obtain the red-shifted and high fluorescence emission; compound 4 and 5 with rigid and strong acceptor unit to promote the fluorescence quantum yield; compound 6 and 7 with low ∆Est values to enhance ROS generation; compound 8-10 with increased steric hindrance to red shift to NIR II emission; compound 11 and 12 with TICT state to boost photothermal conversion property.

Fig. 2. (a) Schematic illustration of MeTPAE with nuclei-targeting function for PDT. Reproduced with permission [59]. Copyright 2022, Wiley-VCH. (b) Schematic illustration of molecular design diagram of a high-performance AIE photosensitizer with cell membrane-targeting function for fluorescence imaging-guided PDT. Reproduced with permission [61]. Copyright 2022, The Royal Society of Chemistry. (c) Schematic illustration of the structure of DBP, TBP, and TBP-SO3. Reproduced with permission [67]. Copyright 2022, The Royal Society of Chemistry. (d) Schematic illustration of the structure of TTT-1, TTT-2, TTT-3 and TTT-4. Reproduced with permission [70]. Copyright 2021, Elsevier. (e) Schematic diagram of the structure of 1,4-dihydropyridine derivatives for both LDs and ER-targeting imaging and therapy. Reproduced with permission [72]. Copyright 2023, Wiley-VCH
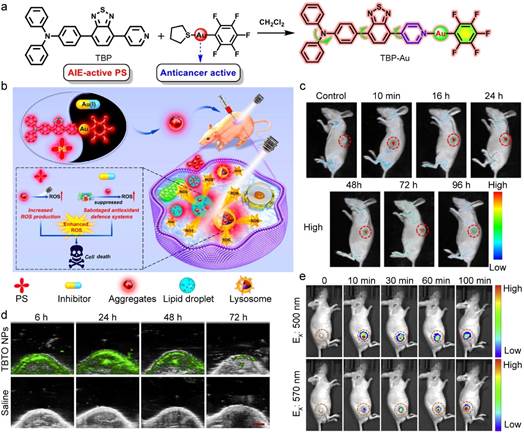
Fig. 3. (a) Molecular design and synthetic route of TBP-Au. (b) Schematic illustration of TBP-Au for anticancer therapeutics through synergistic effects of photodynamic therapy and TrxR inhibition. (c) In vivo real-time fluorescence imaging in HeLa tumor-bearing nude mice after intratumor injection of TBP-Au (λex: 530 nm). Reproduced with permission [85]. Copyright 2021, American Chemical Society. (d) In vivo real-time PA images of the tumor site in the mice after tail intravenous injection with TBTO NPs. (e) In vivo real-time NIR fluorescence imaging in tumor site after intratumoral injection with TBTO NPs. Reproduced with permission [89]. Copyright 2021, Elsevier
6. Conclusion and outlook
In this review, we summarized recent advances of TPA-AIEgens for cancer theranostics. Compared to clinical photoactive agents, TPA-AIEgens shows unique and excellent advantageous such as bright emission, robust ROS generation, high photothermal conversion efficacy, resulting in improved antitumor efficacy. By rational design, TPA-AIEgens demonstrate NIR II absorption/emission, enhanced phototherapy. Owing to AIE characteristics, TPA-AIEgens is available to visualize the tumor foci, which plays a vital role in the clinic diagnosis and image-guided surgeons. Moreover, the PDT and PTT combined with immunotherapy, could be a promising approach to enhance the immunotherapy efficiency. (Table 1).
Despite impressive advancements of TPA-AIEgens in the realm of biomedicine, there are still certain problems and challenges for clinical application. First, TPA-AIEgens suffer from poor targeting capability of tumors and the accumulation of TPA-AIEgens mainly relies on the EPR effect. By modifying the size, shape, structure, surface charge, and target ligands of nanovehicles [113-115], it will be possible to create TPA-AIEgens photoactivators furnishing with unique active targeting 爌roperties for accurate and effective anti-tumor treatment in future. Second, the relationship between structure and target properties of TPA-AIEgens should be systematically studied, which will provide theoretical guidance for further development of the TPA-AIEgens to fulfill clinical requirements. Third, most of the excitation wavelengths of TPA-AIEgens are in the visible range. Compared to those dyes with an absorption in the NIR biological window, TPA-AIEgens encounter the challenges in imaging of deep tissue and high resolution requiremen [116-118]. Besides, TPA-AIEgens, as the photoactive agents, still require rigorous investigation on the therapeutic immunological mechanism and in vivo ADMET (absorption, distribution, metabolism, excretion, and toxicity) properties. Different strategies including the structure design of the probes which have been discussed in section 3, have achieved substantial developments and will definitely promote their transition by addressing the clinical concerns. [108,119]. Last but not least, biosafety issues are inevitable for the development of novel TPA-AIEgens. Biodegradability and long-term toxicity to humans must be considered to meet the demands of clinical translation. Consequently, in order to achieve the therapeutic effect of totally curing malignancies and successfully preventing tumor metastasis and recurrence, it is imperative to conduct in-depth research on TPA-AIEgens through the aforementioned aspects. In future research, the biomedical application of TPA-AIEgens in tumor diagnosis and treatment systems can be vigorously promoted.
Table 1 The summary of TPA-AIEgens for tumor theranostics.
Absorption/ Emission | Target | Diagnosis | Therapeutics | Tumor model | Ref | |
β-TPA-PIO | 405 nm/543nm | Endoplasmic reticulum | FLI | PDT | SKOV3 subcutaneous tumor model | [6] |
tri(3-pyridylphenyl)amine | 405 nm/ 480-580 nm | Nucleus | FLI | PDT | HeLa xenograft tumor model | [58] |
MeTPAE | 405 nm/ 600?0 nm | Nucleus | FLI | PDT | 4T1 subcutaneous tumor model | [59] |
TBMPEI | 465 nm/780 nm | Cell membrane | FLI | PDT | 4T1 subcutaneous tumor model | [61] |
TPANPF6 | 405 nm/ 520-650 nm | Mitochondrion | FLI | PDT | / | [63] |
TPATrzPy-3+ | 405 nm/ 570-650 nm | Mitochondrion | FLI | PDT | Zebrafish model of liver tumor | [64] |
T-BDP | 363, 631 nm/ 724 nm | Lysosome | FLI | PDT+PTT | / | [69] |
DTPAP-P | 456 nm/543 nm | Nucleus | FLI | PDT | / | [71] |
TPA-DHPy-Py | 488 nm/ 600-700nm | Endoplasmic reticulum and lipid droplets | FLI | PDT | MDA-MB-231 subcutaneous model | [72] |
BDPTPA | 808 nm/- | Tumor | PAI | PDT+PTT | 143B xenograft tumor model | [74] |
TPA-T-TQ | 780 nm/- | Tumor | PAI | PTT | 4T1 subcutaneous tumor model | [75] |
Ce6@HA-Cys-TTDTT | 635 nm/ 700-750 nm | Tumor | NIR FLI | PDT+PTT | HeLa subcutaneous tumor model | [76] |
TPA-DPPy | 405 nm/- | Saturated fatty acids and mitochondrion | FLI | PDT | / | [82] |
TBP-Au | 488, 870 nm/ 575-630nm | Lysosome and lipid droplets | 2P- FLI | PDT | HeLa subcutaneous tumor model | [85] |
TPA-2PI | 960 nm/ 600-800 nm | DNA and?mitochondrion | 2P- FLI | PDT | 4T1 subcutaneous tumor model | [86] |
CyQN-BTT | 671, 800 nm/ 900-1100 nm | Tumor | NIR-II FLI+PAI | PDT+PTT | 4T1 subcutaneous tumor model | [91] |
MeO-TPA-TPP | 455 nm/ 550-620 nm | Mitochondrion | FLI | PDT | 4T1 breast tumor model | [97] |
TPA-DHPy | 488 nm/ 500-650 nm | Endoplasmic reticulum and lipid droplets | FLI | PDT | HeLa subcutaneous tumor model | [98] |
PQ-TPAOC1 NPs | 660 nm/- | Tumor | / | PDT+PTT | 4T1 tumor model | [105] |
TPA-TDPP | 638 nm/743 nm | Tumor | FLI | PDT+PTT | HeLa tumor model | [106] |
tTDCR | 488 nm/627 nm | Tumor | FLI | PDT+immuno- therapy | 4T1 subcutaneous tumor model | [109] |
TPA-DCR | 460 nm/ 620?5 nm | / | FLI | PDT+immuno- therapy | 4T1 subcutaneous tumor model | [110] |
α-Th-TPA-PIO | 465 nm/660 nm | Endoplasmic reticulum | FLI | PDT+immune- therapy | B16-F10 subcutaneous tumor model | [111] |
Platinum(II) Triphenylamine | 405 nm/ 620 ?20 nm | Mitochondrion | FLI | PDT+immune- therapy | U14 subcutaneous tumor model | [112] |
DCP-PTPA | 680 nm/- | Tumor | PAI | / | 4T1 xenograft tumor model | [120] |
BOPHY-2TPA | 561 nm/ 630-690 nm | HeLa cell | FLI | PDT | / | [121] |
?/span>
https://blog.sciencenet.cn/blog-2438823-1427296.html
上一篇:Fluorescent Probe forRatiometric Monitoring of Peroxynitrite