博文
[转载]异戊二烯化:探索蛋白修饰的奥秘
||
引言
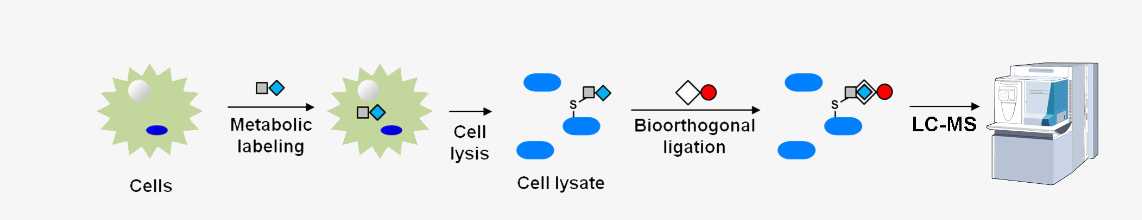
G蛋白是重要的信号分子,负责将七螺旋跨膜受体传递的信号传导给下游效应器。G蛋白定位在细胞质膜上对其功能至关重要,而异戊二烯化(prenylation)在这一过程中扮演着重要角色[1]。异戊二烯化是一种翻译后修饰,涉及将脂质分子添加到蛋白质C-末端附近的半胱氨酸残基上。脂质分子可以是顺式法尼醇(farnesyl)或戊烯酰基(geranylgeranyl),添加的脂质类型取决于具体被修饰的蛋白质。在G蛋白中,γ亚单位被异戊二烯化修饰[1]。
异戊二烯化在G蛋白组装与功能中的作用
G蛋白的亚单位相互结合形成三聚体是其与受体相互作用所必需的。G蛋白γ亚单位(G γ)经历了一系列羧基端加工事件,包括半胱氨酸异戊二烯化、蛋白质水解和甲基化。最新的研究进展揭示了G γ亚单位的翻译后修饰对G蛋白三聚体的组装、膜结合和功能所起到的作用。这些修饰对于重要的蛋白质-蛋白质相互作用和膜-蛋白质相互作用都是必需的[1]。
在CAAX模体之外的区域会影响G蛋白γ亚单位的异戊二烯化的特异性。重组G蛋白γ亚单位的体外加工对于活化βγ复合物的组装是必需的[1]。
尽管G γ亚单位的异戊二烯化和/或进一步的羧基端加工对于βγ复合物的组装并非必需,但这些修饰对于βγ的功能是不可或缺的。βγ与G蛋白α亚单位或效应分子的高亲和性相互作用要求γ亚单位被异戊二烯化修饰。为了研究γ亚单位羧基端加工的作用,使用了一种不能发生异戊二烯化和进一步羧基端加工的突变体β1γ2(β1γ2(C68S))[2]。
G蛋白β1γ2亚单位促进微管的组装。βγ与G蛋白α亚单位或效应分子的高亲和性相互作用要求γ亚单位被异戊二烯化修饰。此研究还表明,异戊二烯化在介导βγ与其他蛋白质之间的相互作用中可能起着关键作用[2]。
进展与发展
近年来的研究集中在蛋白质异戊二烯化的分子机制及其在G蛋白功能中的作用上。已知的异戊二烯化蛋白包括真菌交配因子、细胞核骨架蛋白、Ras和与Ras相关的GTP结合蛋白(G蛋白)的亚单位等。这些蛋白质的异戊二烯化对于它们的功能至关重要,而异戊二烯化缺陷与多种疾病有关,包括癌症、心血管疾病和神经系统疾病[3]。
异戊二烯化在不同领域中的应用
异戊二烯化在药物发现、癌症研究和神经生物学等多个领域具有重要应用。在药物发现领域,已开发出抑制异戊二烯化的药物作为潜在的抗癌剂。这些抑制剂靶向负责蛋白质异戊二烯化的酶,且在临床前研究中显示出良好的前景[3]。
在癌症研究中,异戊二烯化抑制剂已被用于研究异戊二烯化在癌细胞生长和存活中的作用。这些抑制剂已被证明能够诱导癌细胞凋亡,并正在作为潜在的抗癌治疗药物进行研究[3]。
在神经生物学中,异戊二烯化被认为参与突触功能和可塑性的调节。异戊二烯化抑制剂已被用于研究异戊二烯化在突触传递中的作用,并在阿尔茨海默病和精神分裂症等神经系统疾病的治疗中显示出潜力[3]。
结论
异戊二烯化在G蛋白组装与功能中起着重要作用,异戊二烯化缺陷与多种疾病相关。近年来的研究集中在蛋白质异戊二烯化的分子机制及其在G蛋白功能中的作用上。异戊二烯化抑制剂在药物发现、癌症研究和神经生物学等多个领域具有重要应用。
Citations:
[1] Higgins, J. B., & Casey, P. J. (1996). The role of prenylation in G-protein assembly and function. Cellular signalling, 8(6), 433-437.https://pubmed.ncbi.nlm.nih.gov/8958445/
[2]Roychowdhury, S., & Rasenick, M. M. (1997). G protein β1γ2 subunits promote microtubule assembly. Journal of Biological Chemistry, 272(50), 31576-31581. https://www.jbc.org/content/272/50/31576.full.pdf
[3] Zhang, F. L., & Casey, P. J. (1996). Protein prenylation: molecular mechanisms and functional consequences. Annual review of biochemistry, 65(1), 241-269.https://www.annualreviews.org/doi/abs/10.1146/annurev.bi.65.070196.001325
## Introduction
G-proteins are important signaling molecules that transduce signals from heptahelical transmembrane receptors to downstream effectors. The localization of a G-protein to the plasma membrane is essential for its function, and prenylation plays a crucial role in this process[1]. Prenylation is a post-translational modification that involves the addition of a lipid molecule to a cysteine residue near the C-terminus of the protein. The lipid molecule can be either a farnesyl or a geranylgeranyl group, and the type of lipid added depends on the specific protein being modified. In the case of G-proteins, the gamma subunit is prenylated[1].
## Role of Prenylation in G-Protein Assembly and Function
The association of a G-protein's subunits to form its trimer is required for interaction with its receptor. The G-protein gamma subunits (G gamma) are subject to a set of carboxyl-terminal processing events that include prenylation of a cysteine, proteolysis, and methylation. Recent advances have elucidated the contributions that the post-translational modifications of the G gamma subunit have on the assembly, membrane association, and function of the G-protein trimer. These modifications are required for important protein-protein, in addition to membrane-protein, interactions[1].
Regions outside of the CAAX motif influence the specificity of prenylation of G protein gamma subunits. In vitro processing of recombinant G protein gamma subunits is required for assembly of an active beta gamma complex[1].
Although prenylation and/or further carboxyl-terminal processing of G gamma is not required for assembly of beta gamma complexes, such modifications are indispensable for the function of beta gamma. High affinity interactions of beta gamma with either G protein alpha subunits or effector molecules require that the gamma subunit be prenylated. To examine the role of the gamma subunit carboxyl-terminal processing, a mutant beta1gamma2, beta1gamma2(C68S), which cannot undergo prenylation and further carboxyl-terminal processing was used[2].
G protein beta1gamma2 subunits promote microtubule assembly. High affinity interactions of beta gamma with either G protein alpha subunits or effector molecules require that the gamma subunit be prenylated. Furthermore, it appears from this study that prenylation may play a crucial role in mediating the association between beta gamma and other proteins[2].
## Progress and Development
Recent studies have focused on the molecular mechanisms of protein prenylation and its role in G protein function. Known prenylated proteins include fungal mating factors, nuclear lamins, Ras and Ras-related GTP-binding proteins (G proteins), the subunits of trimeric G proteins, and others. The prenylation of these proteins is essential for their function, and defects in prenylation have been linked to a variety of diseases, including cancer, cardiovascular disease, and neurological disorders[3].
## Applications in Different Fields
Prenylation has important applications in various fields, including drug discovery, cancer research, and neurobiology. In drug discovery, prenylation inhibitors have been developed as potential anticancer agents. These inhibitors target the enzymes responsible for protein prenylation, and have shown promise in preclinical studies[3].
In cancer research, prenylation inhibitors have been used to study the role of prenylation in cancer cell growth and survival. These inhibitors have been shown to induce apoptosis in cancer cells, and are being investigated as potential cancer therapeutics[3].
In neurobiology, prenylation has been implicated in the regulation of synaptic function and plasticity. Prenylation inhibitors have been used to study the role of prenylation in synaptic transmission, and have shown promise in the treatment of neurological disorders such as Alzheimer's disease and schizophrenia[3].
## Conclusion
Prenylation plays a crucial role in G-protein assembly and function, and defects in prenylation have been linked to a variety of diseases. Recent studies have focused on the molecular mechanisms of protein prenylation and its role in G protein function. Prenylation inhibitors have important applications in various fields, including drug discovery, cancer research, and neurobiology.
Citations:
[1] Higgins, J. B., & Casey, P. J. (1996). The role of prenylation in G-protein assembly and function. Cellular signalling, 8(6), 433-437.https://pubmed.ncbi.nlm.nih.gov/8958445/
[2]Roychowdhury, S., & Rasenick, M. M. (1997). G protein β1γ2 subunits promote microtubule assembly. Journal of Biological Chemistry, 272(50), 31576-31581. https://www.jbc.org/content/272/50/31576.full.pdf
[3] Zhang, F. L., & Casey, P. J. (1996). Protein prenylation: molecular mechanisms and functional consequences. Annual review of biochemistry, 65(1), 241-269.https://www.annualreviews.org/doi/abs/10.1146/annurev.bi.65.070196.001325
https://blog.sciencenet.cn/blog-3402731-1393526.html
上一篇:[转载]"从分子到应用:探索蛋白质戊烯酰化的异戊二烯化奇迹"
下一篇:RNP介导的基因组编辑:解锁基因功能的新工具